Overdischarge in Li-ion Batteries with different anodes
- Baba Mulani

- Jun 5, 2024
- 2 min read
Overdischarge is still a pivotal challenge in lithium-ion batteries, which particularly affects the battery's long-term functionality & safety. For cells with a graphite-based anode, overdischarging beyond a certain depth of discharge (DOD%), specifically greater than 110%, results in irreversible damage.
This phenomena was rigorously examined in one of the studies that explored the repercussions of overdischarge with two different anode materials: graphite-based & Lithium Titanate Oxide (LTO).
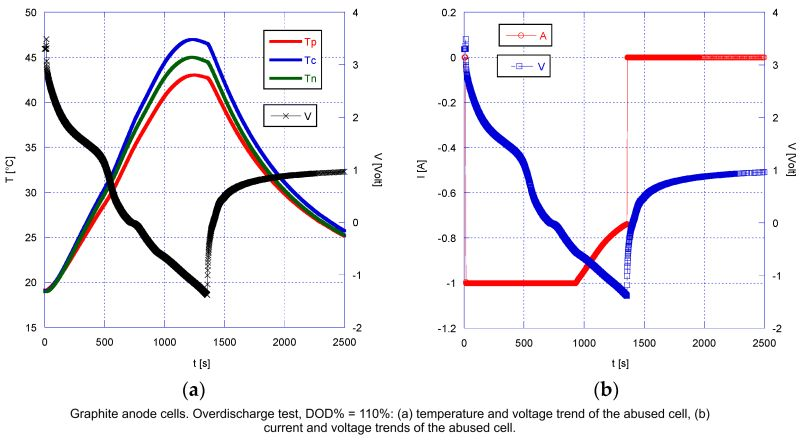
The study by Minale C et al outlined that when graphite anode cells are subjected to overdischarge beyond a DOD% of 110%, the damage manifests in several forms: the formation of electrolyte deposits on the anode & notable structural changes at the cathode, both of which impede the battery's ability to recharge. At any level of overdischarge, the deposition of copper was observed on the anode, suggesting the dissolution of the copper current collector & subsequent deposition during recharge cycles. This phenomenon can lead to internal shorts, which exacerbate the degradation of the cell.
In contrast, cells with an LTO-based anode demonstrated considerable resilience against overdischarge. These cells could generally be recharged & returned to operational status, even when overdischarged to a DOD% of 140%. However, it was noted that such deep overdischarge significantly reduced the recoverable capacity of these cells.
For graphite cells, a critical observation was that beyond a certain threshold of overdischarge, the risk of polarity inversion was also noted, which is often a precursor to cell failure. This inversion was directly associated with the depth of discharge & was not observed in LTO anode cells.
The graph shows that the cell’s voltage initially decreases to a minimum of −1.53 V when the DOD reaches 113%. After this point, the voltage begins to recover, eventually stabilizing around −0.5 V despite the battery continuing to discharge at a constant current of −1 A. This recovery in voltage, often referred to as a "self-restoring" effect, suggests some reversibility in the cell's behavior under extreme overdischarge conditions.
Polarity inversion, where the cell voltage drops below zero, was consistently observed at a DOD% of around 106%. This phenomenon is critical, as it typically signals the start of detrimental changes within the cell. However, permanent damage & failure are more specifically associated with an inversion in the voltage trend, noted to occur when the DOD% is between 110–113%.